|
Find the latest updates on all areas of the ORC, including the IACUC, the NTR IRB,
RCOI, and International Compliance.
This quarter we introduce our ORC member – Christina Aguilar!
In this issue, we’ll help you get to the “Yes” with your Research Conflict of Interest training and disclosure form.
In this issue, we answer questions about how to make modifications to your research
protocols for both IRB and IACUC approved projects.
In this special article, we detail the North Texas Regional IRB’s experience at JPS’
annual Research & Quality Symposium.
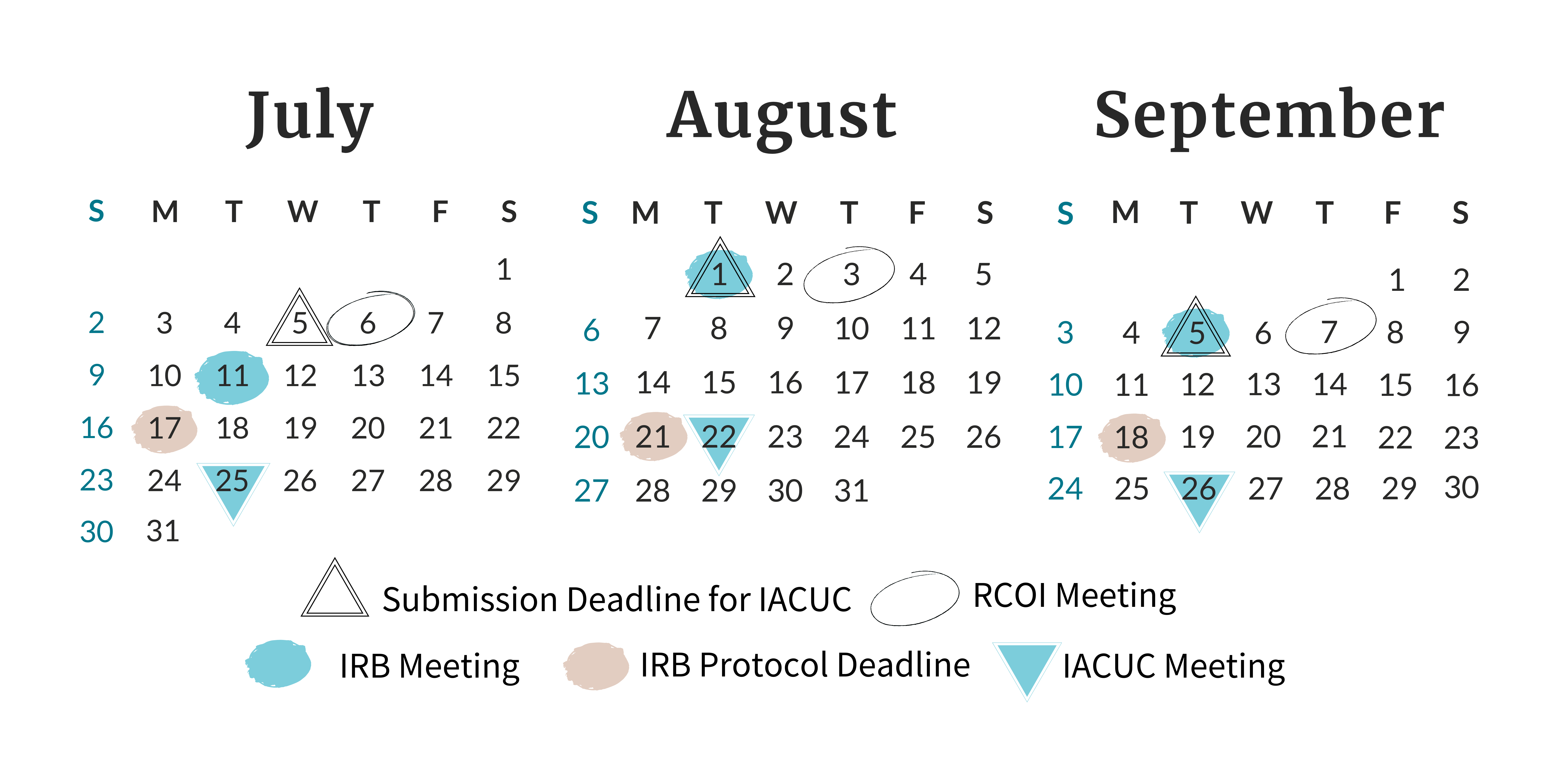
Find all of the meeting and deadline dates for the next three months of Board and
Committee meetings.
|
|
What’s Happening in the ORC?
Here is the latest news from the ORC:
- We would like to congratulate Christina Aguilar who was promoted from her previous
role as the Senior IACUC Administrator to her current role as Assistant Director,
IACUC!
- In May, the NTR IRB had the privilege of participating in the JPS Annual Research
and Quality Symposium. Please see our special article below for an in-depth look at our contribution to the symposium!
- The NTR IRB has created a form on their website for submitting Reliance Agreement
requests titled “NTR IRB Reliance Agreement.” Investigators who are engaged in a collaborative study can use this form to request
for another IRB to be the “IRB of record” for a multi-site human subject research
study, or vice versa (request the NTR IRB to be the IRB of record for a multi-site
human subject research study). Researchers requesting reliance should first complete
this form, which will request an explanation of the situation/request, the reason
for the reliance agreement, and any required documents.
- We are gearing up for our Annual RCOI Disclosure Campaign! Please be sure to check
your emails for updates regarding this year’s campaign, which is set to start on September
1st, 2023.
back to top
Employee Spotlight – Meet Christina Aguilar
How long have you worked in the UNT HealthOffice of Research Compliance? What is your
role, and what do you like best about it? I have worked in the Office of Research Compliance since March of 2016, so that is
about 7 years. I am the Assistant Director for the IACUC. I’ve worked in this industry
for close to 20 years, and being an advocate for animals is by far one of the best
parts of the job. Plus, the team I get to work with in the Office of Research Compliance
makes it worth coming to work every day.
Of the following values, which one do you believe you exemplify the most, and why?(Courageous Integrity, Be Curious, We Care, Better Together, and Show Your Fire!) Better Together! At UNTHSC, I really like the collaborative approach we take in ensuring the welfare
of our animals. I love learning about each investigator’s research. I enjoy working
with DLAM, they really take great care of the animals. We have the best Institutional
Animal Care and Use Committee! I may be a little biased, but our IACUC Chair is wonderful
to work with, and each committee member takes their role seriously. Individually,
we all do our jobs well, but together we bring the excellence of an exemplary Animal
Care and Use program. This was noted in our recent accreditation visit, when the site
visitors commented on how well we worked together. We really are better together!
What is something we would be surprised to find out about you? I’m not sure if it’s too surprising, but when I’m not fighting for the welfare of
animals, I enjoy creating music! Whether it’s pouring out my soul in my cello, banging
out tunes on the piano, writing songs on my acoustic guitar, or jamming out on my
bass guitar – I find some sort of way to express myself through music.
back to top
How to Get to the Yes
Research Conflict of Interest: What’s a Researcher to do?
Research Conflict of Interest, or “RCOI”, refers to situations in which financial, or other personal considerations may compromise, or
have the appearance of compromising a researcher’s professional judgement in the conduct or reporting of research.
So, what does this actually mean?
Basically, conflicts of interest in research come in a variety of different shapes,
sizes, and flavors. Consider the following situations (for the purposes of these examples,
all situations take place in the U.S.):
|
|
|
- Dr. Bruce Banner, who is faculty at Stark University, has developed a new technology
to help individuals recovering from knee replacement surgery. The patent for this
technology is under the company, Strange Corporation, Inc., and Dr. Banner is the
founder and CEO (note that Strange Corporation itself is not affiliated with Stark
University). Dr. Banner will also be setting up a pilot human subject research project
to test the safety and effectiveness of the technology, and he will be recruiting,
consenting, and enrolling subjects from Stark University as well as the surrounding
community. The ultimate goal will be to market this technology to the general population.
- Ms. Dany Targaryen serves as a Co-Investigator and Research Coordinator for several
research projects at her place of employment, Winterfell State College. Separately,
Ms. Targaryen also serves as an officer on the Board of a company called Dragon Solutions,
Inc., which provides analytical services for research studies. Ms. Targaryen does
not receive any compensation from Dragon Solutions for her time on the Board. Additionally,
one of the research studies that Ms. Targaryen is listed on may, in the near future,
be using Dragon Solutions to help conduct some of the analyses for their project.
- Dr. Bruce Wayne (a faculty and researcher at the University of Arkham) is named on
a PHS -funded project and is a member of the American Psychological Association and
Chair of the Steering Committee. His travel is reimbursed by the American Psychological
Association to attend a committee meeting.
- Neurologist, Dr. Wanda Maximoff, recently started as a physician-researcher at Wakanda
General Hospital (WGH). WGH is involved in an industry-sponsored Phase III clinical
trial (as a recruiting site) which is studying the effects of a new drug to help individuals
with insomnia. Dr. Maximoff is not the site PI, however, she is a co-investigator
on the study. Prior to coming to WGH, Dr. Maximoff worked at the company, within the
team, that developed the insomnia drug being currently studied as part of this clinical
trial.
Each of the above examples present a potential (or actual) research conflict of interest
that will need to be reviewed by the RCOI office.
While there are federal regulations that provide guidelines for ensuring objectivity
in research (see 42 CFR Part 50, Subpart F) and ensuring institutions appropriately manage conflicts of interest (see 45 CFR Part 94.4), it is up to the researchers and their institutions to ensure any potential RCOIs
(whether they be financial or not) are appropriately reviewed and managed (as needed).
To help with that, UNT Healthresearchers are always able to review the UNT Healthpolicy 8.105 on Research Conflict of Interest as well as the UNT HealthRCOI Standard Operating Procedures.
Here is how the UNT HealthRCOI disclosure process works:
- At HSC, researchers are required to submit an annual RCOI disclosure form. The RCOI
campaign typically runs from September 1 – October 1 each year.
- RCOI training also needs to be completed annually, at the time the researcher completes
their disclosure form (current RCOI training is done in the CITI Program system).
- Through the rest of the year, researchers should disclose any new significant financial
interests (SFIs, e.g., new grant/award received or relationship in which the researcher
will be paid for time services, etc.), travel disclosures for reimbursed or sponsored
travel (related to institutional responsibilities), or any other situations in which
the investigator will be devoting time or energy to an external entity/project which
may bias (or potentially bias) the researcher’s work at HSC.
- The new/updated disclosures should be provided within 30 days of discovering or acquiring
a new SFI or other potential conflict of interest, or sponsored travel (note this
only applies to investigators with PHS funding).
- Upon receipt of a new or updated disclosure from a researcher, the RCOI Office will
take an initial look at the disclosure and ensure all appropriate information has
been received (the RCOI Office may follow-up with a researcher as needed, to ensure
all necessary information is received).
- Once the pre-review of the disclosure has been completed, the RCOI Committee will
either determine that:
- No further action is needed on the disclosure, or;
- That an RCOI Management Plan will need to be implemented. In this case, the RCOI Office
and Committee will work with the researcher to create and implement the Management
Plan. This Management Plan will be reviewed on at least an annual basis.
If you need additional information regarding the RCOI process at HSC, please visit
the UNT HealthOffice of Research Compliance RCOI webpage, or contact us at Research.Compliance@unthealth.edu or 817-735-0409. We are always happy to answer any questions as needed!
back to top
|
|
|
You’ve Got Questions, We’ve Got Answers!
Q: I need to submit a modification to my study. What do I need to do?
For Human Subject Research Studies: All requests for modifications to IRB-approved human subject research studies must
be submitted to the North Texas Regional IRB within the IRBNet system. Examples of
protocol modifications include key personnel changes, revisions to the study procedures,
adding new study documents, adding a recruitment source, etc.
How is a request for a protocol modification submitted for review? To submit a request for a protocol modification:
- First, access the project you want to modify in IRBNet.
- Create a new package for the submission. To create a new package, click on the link
in the left-hand toolbar that reads, “Create a New Package.” You will select “Amendment/Modification”
for package type.
- Upload all relevant documents (see the details of which materials need to be submitted
below).
- Once all of the documents have been uploaded, submit the package in IRBNet. To do
this, click on the “Submit Package” button.
What documents need to be submitted when requesting a protocol modification?
Key Personnel Changes Only: If only key personnel changes are being requested (e.g., addition and/or removal of key personnel),
then the following documents need to be submitted for review:
- A completed “Application for Change in Study Personnel” form that has been signed
by the principal investigator (PI), OR a signed memo from the PI that lists the requested changes.
- Both tracked and clean versions of the updated protocol synopsis displaying the revised
list of key personnel; Note: Studies that require continuing reviews may wait until the time of continuing
review to update the protocol synopsis.
- If any new key personnel are being added to the study, the following documents must also be
submitted:
- Evidence that each new key personnel has completed adequate training in human subject
research (e.g., CITI);
- A signed conflict of interest (COI) form for each new key personnel. Note: COI forms are required for Expedited and Full Board studies only (COI forms
are not required for Exempt studies).
Protocol Modifications: When requesting other types of protocol modifications, the documents (listed below)
need to be submitted for review:
- A signed memo from the PI that describes the proposed modifications and a brief justification
for each of the changes.
- A “tracked changes” (redline) copy of the revised research document(s) (e.g., protocol
synopsis, consent form, etc.) reflecting the proposed modifications. Note: Researchers should use the most-recent, IRB-approved versions of the research
documents when making changes.
- A “clean” copy (where all tracked changes have been accepted) of the revised research
document(s) reflecting the proposed modifications.
- Other research-related document(s), if any, that are associated with the proposed
modifications (e.g., new research documents that are being added to the study, etc.).
Reminder: A protocol modification cannot be implemented until after the North Texas Regional IRB has approved the modification (and the PI/study team
has received notification of the approval). Implementing the changes before IRB approval
will result in a protocol violation or finding of non-compliance.
For Animal Research Studies: If you need to make changes to your approved IACUC protocol, you will do so by creating
an Amendment in GRAMS. Examples of amendments include changes in funding, changes
in personnel, revisions or additional procedures or study timepoints, changes in animal
model, or increase in animal numbers.
How to submit an amendment for review: To submit an amendment to an IACUC protocol:
- Log into GRAMS, click the IACUC tab, and navigate to your Research Team space.
- Select the protocol you would like to amend.
- Click the Create Amendment button under the Next Steps section of the left toolbar; this will take you into the amendment form.
- In the “Amendment Short Title”, be sure this section includes your IACUC Protocol
Number.
- For “Describe the Changes”, provide the details of the amendment. If increasing animal
numbers, be sure to list the number of animals being added. If changing personnel,
be sure to list the personnel names.
- In the “Describe the Rationale for Changes” section, please provide a justification
for the need to amend the protocol.
- When “Continue” is clicked, it takes you into the original protocol. Be sure to only
edit the sections that will be affected by the changes made.
- Once all the changes have been made, hit “Save” and then “Exit” (this will take you
out of the form). Please note that this does not automatically submit the amendment
for review.
- After exiting the protocol form, navigate to the left menu, where you will find the
submit button.
Amendment Review: Once the amendment is submitted, the IACUC Office performs a pre-review of the amendment.
If edits are needed, this will then be communicated back to you through the GRAMS
system. There are four types of Reviews for amendments, determined by the types of
changes made to the protocol:
- Full Committee Review (FCR): Generally, these are amendments that are adding significant changes, for example
changing the objectives of the study, adding procedures that involve unrelieved pain/
distress, or adding a USDA covered species.
- Designated Member Review (DMR): These amendments are sent electronically to the Committee, who are given 5 business
days to call for Full Committee Review. If no one calls for Full Committee Review,
the amendment is then assigned to designated reviewer(s) (i.e., IACUC Members) to
review, approve, require modifications, or call for Full Committee Review. Examples
of these types of amendments include increasing animal numbers >10%, adding hazardous
substances, or adding a survival surgery.
- Veterinary Verification and Consultation (VVC): These amendments are reviewed by the veterinarian. Examples of amendments that qualify
for VVC are increasing animal numbers <10%, changes in anesthesia, addition of euthanasia
method within the AVMA Guidelines for euthanasia, changes in approved blood/fluid/tissue
collections, addition of non-survival surgery.
- Administrative Review (AR): These are amendments that are reviewed by the IACUC Office. Examples of these types
of amendments are changes in funding, changes in personnel, or administrative edits/clarifications
in the protocol.
Reminders and Tips & Tricks for IACUC amendments:
- Please note that as the amendment opens the protocol document in GRAMS for editing,
you may only have one amendment open at a time; as such, you will not be able to submit
a second amendment until the open amendment’s review process is complete.
- If adding personnel, be sure that all personnel being added have completed all training
and personnel requirements before submitting the amendment. This will help prevent
delays in the review and approval process.
- If adding public funding (e.g., NIH), be sure to attach the vertebrate animal section
and the Research Strategy Section.
- If adding a substance and/or a procedure, be sure to create a procedure and attach
it to the experiment section.
- If adding animal numbers, be sure to make the edits in the experiments section. The
Animal Justification table should then be automatically updated.
- If adding a laboratory that has not yet been approved for animal use, be sure to set
up a time for the IACUC to inspect the laboratory for approval.
- If adding hazardous substances, be sure to obtain any necessary safety approvals,
attach the hazardous agent attachment to the protocol, and conduct consults with Safety
and DLAM.
- Remember not to initiate any of the changes until IACUC approval has been granted
(and the PI/study team has received notification of the approval). Initiating changes
in your study without IACUC approval of the modifications can result in protocol non-compliance.
We are here to help you succeed! We hope this section will help you throughout your
submission process.
Have a question you would like answered? Just click the link below to submit your
question.
back to top
|
|
|
The North Texas Regional IRB at the May 2023 JPS Symposium!
As some may already know, the John Peter Smith (JPS) Health Network’s Office of Clinical
Research, Quality Division, and Department of Academic Affairs hosted their annual
Research & Quality Symposium on May 18th (in-person) and May 19th (virtually). The in-person event was held at the Texas Christian University (TCU)
Horned Frog Grand Ballroom, located in Brown-Lupton University Union, and included
the opportunity to network, view posters from selected research projects, and to watch
all the Academic Excellence, Research, and Quality Awards be presented to some of
the many talented individuals at JPS. A memorable Keynote Address was given by Dr.
Brett Giroir, a physician-scientist (and former Assistant Secretary for Health in
HHS, Acting FDA Commissioner, and Admiral in the US Public Health Service Commissioned
Corps) who described his experience at the front lines of the COVID-19 response as
a member of the White House Coronavirus Task Force and the national lead for testing
and diagnostics (“Testing Czar”).
The North Texas Regional (NTR) IRB was invited to, and had the pleasure of, hosting
a booth at the Symposium. This allowed for an invaluable experience to meet with many
of our JPS researchers and partners face-to-face, and also connect potential research
collaborators from JPS, HSC, as well as TCU! Our booth included general information
on what the NTR IRB is (i.e., a regional IRB that serves many different organizations
in North Texas area, allowing a simplified landscape for researchers to collaborate
on research projects!), and also included a plethora of guidance and resources to
put researchers on the right path to submitting their human subject research protocols!
There is no doubt the event was a hit – countless people from a wide variety of areas
stopped by throughout the networking portion of the evening. JPS physicians, residents/interns,
researchers, staff nurses and staff, TCU leadership, researchers, staff and students,
and colleagues and researchers from UNT Healthwere just some of the few who stopped
by to ask questions, discuss their potential or current research projects, or just
visit with fellow colleagues (there were even a few guest appearances by some of our
beloved NTR IRB members!). We could not have been happier to have had the opportunity
to be involved in this event! Our goal at the NTR IRB is to “protect research participants,
serve the community, and enhance the human research enterprise of its collaborating
partners”; however, we rarely get to see the results of the research we approve and
the advancements being made by our brilliant research community – and this event allowed
us the opportunity to do just that!
Take a look below at some of the snapshots captured from the in-person event! Additionally,
for more details about the 2023 JPS Research & Quality Symposium, please visit the
website at https://www.jpshealthnet.org/research-quality-symposium.
 

back to top
|